Methodical Data Alignment: An Overlooked Data Cleansing Step – Part 2
Dive into two more use cases where accurate data alignment can help identify accurate process insights and yield valuable process savings.
Our last blog post discussed why accurate data alignment can be key to identifying accurate process insights and yielding valuable process savings. In this edition, we’ll dive into two more use cases where data alignment is often overlooked to further demonstrate this point.
An extremely common use case is to compare process metrics before and after some process event. The process event could be anything of interest, such as a process unit restart, equipment or control strategy modifications, or process experimentation The analysis begins by identifying the process events and then calculating the metrics over appropriate time ranges before and after each event. The alignment step typically involves creating a common time basis spanning before/after operation and moving the before/after calculated metrics to the same point in time for comparison. Seeq Formula’s capsule adjustment functions are invaluable for creating a common time basis, and the Signal from Condition tool is commonly used to find a value within a time period (condition) and move it to a specific location in time.
These methods are illustrated in Figure 4 for before/after pressure averages (process metrics) calculated around each equipment maintenance (identified process event) that occurred over a one month period. Tabular results are included in Figure 5.
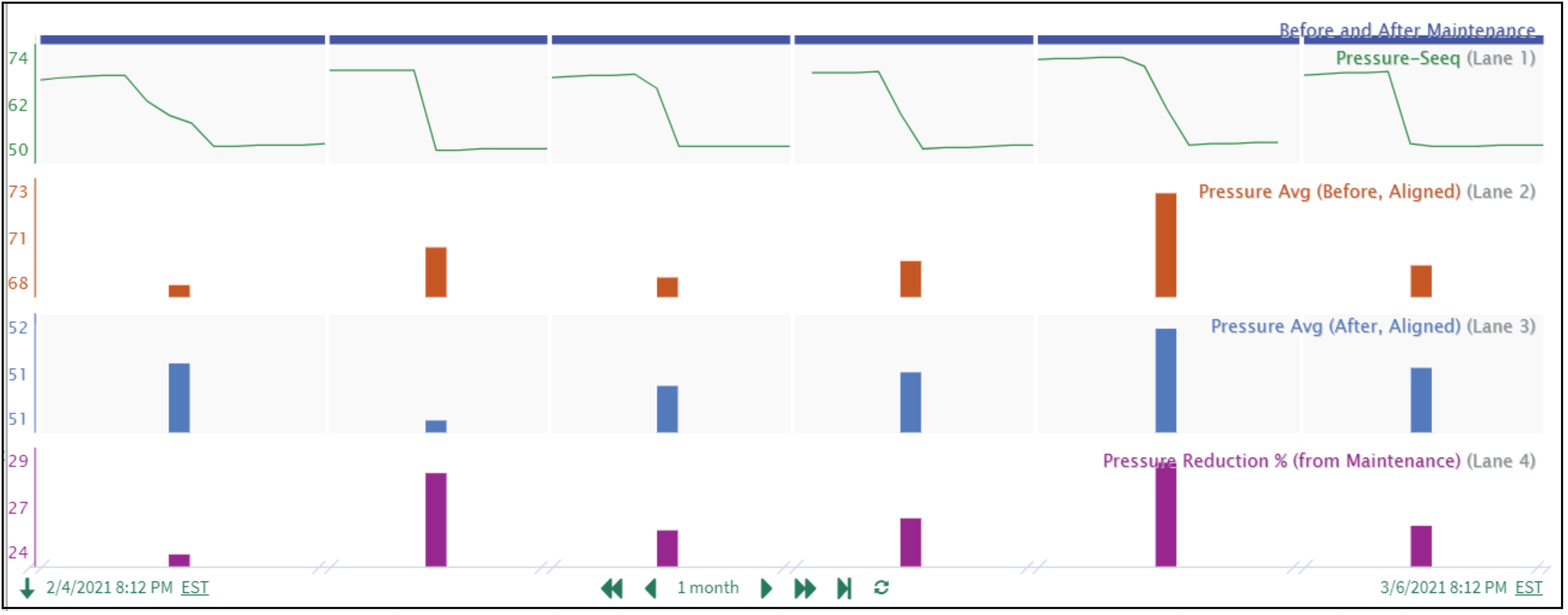
The ability to quickly calculate process metrics before/after process events, and compare them via data alignment, is a valuable tool for the process engineer often tasked with designing and evaluating process changes to remedy issues or further optimize operation.
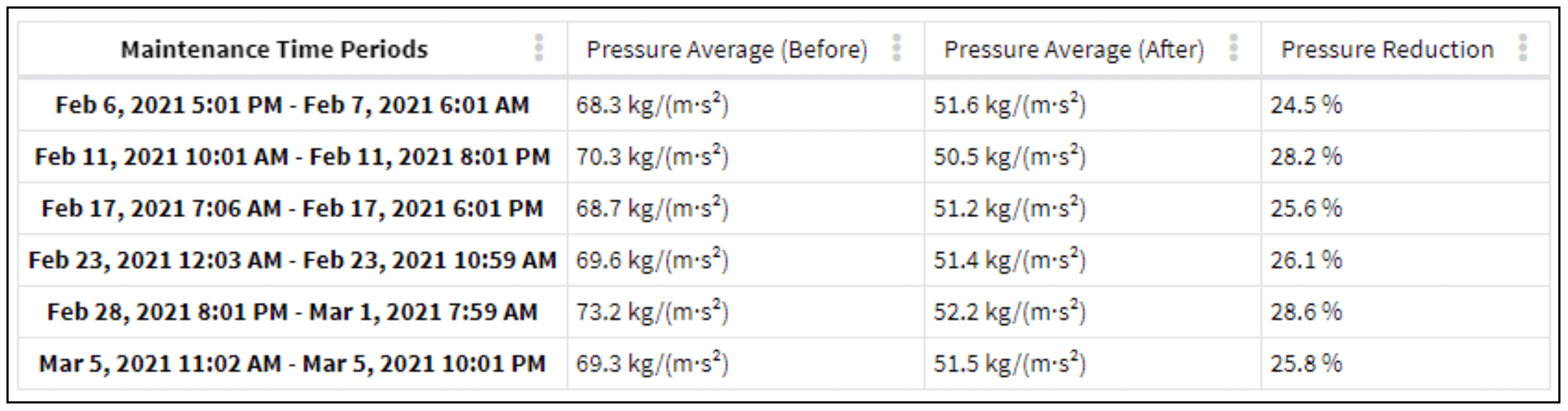
The ability to quickly calculate process metrics before/after process events, and compare them via data alignment, is a valuable tool for the process engineer often tasked with designing and evaluating process changes to remedy issues or further optimize operation.
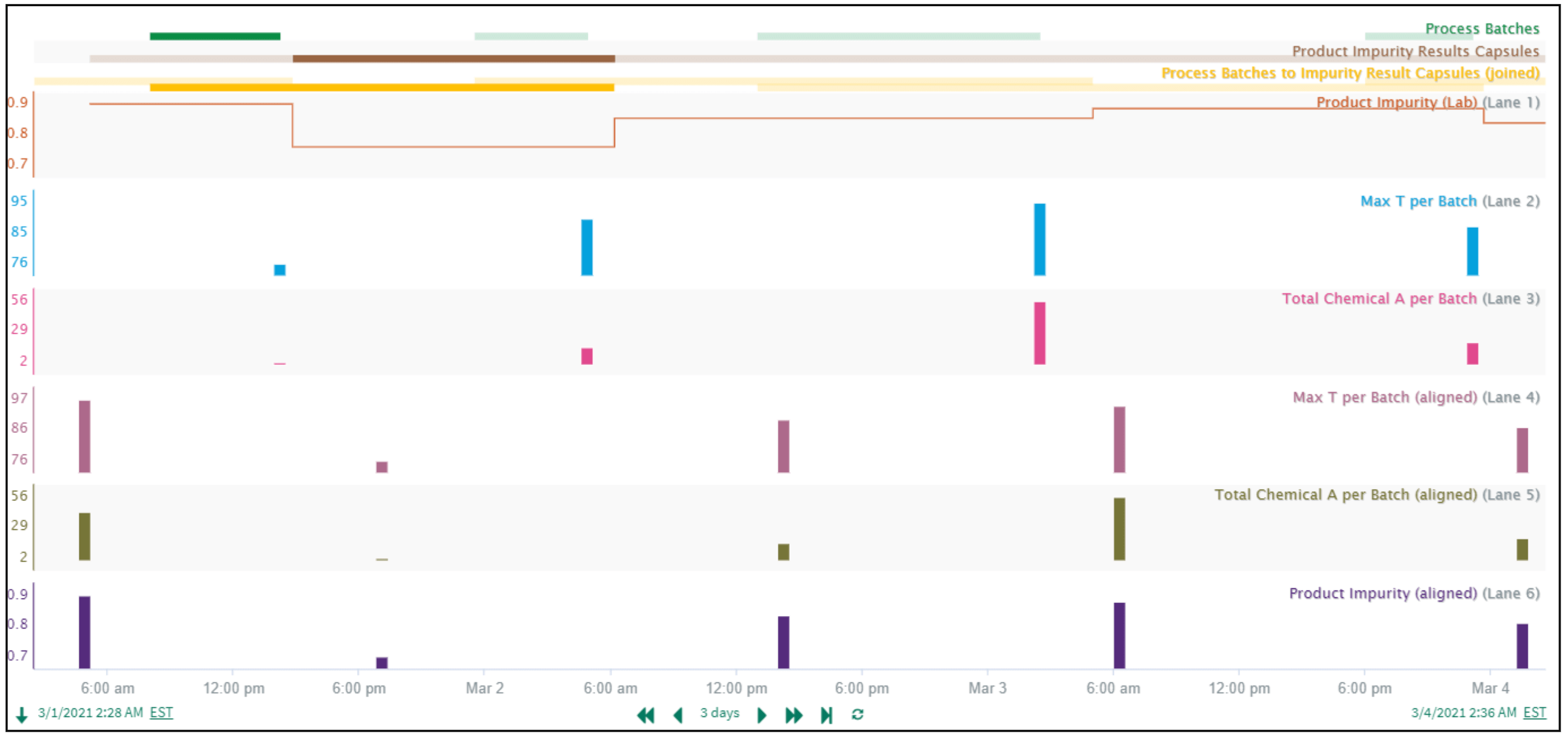
In this example, we align process values with lab results. The “Product Impurity” lab results (Figure 6) are reported at varying time intervals following batch completion, so a constant time delay alignment approach isn’t feasible.
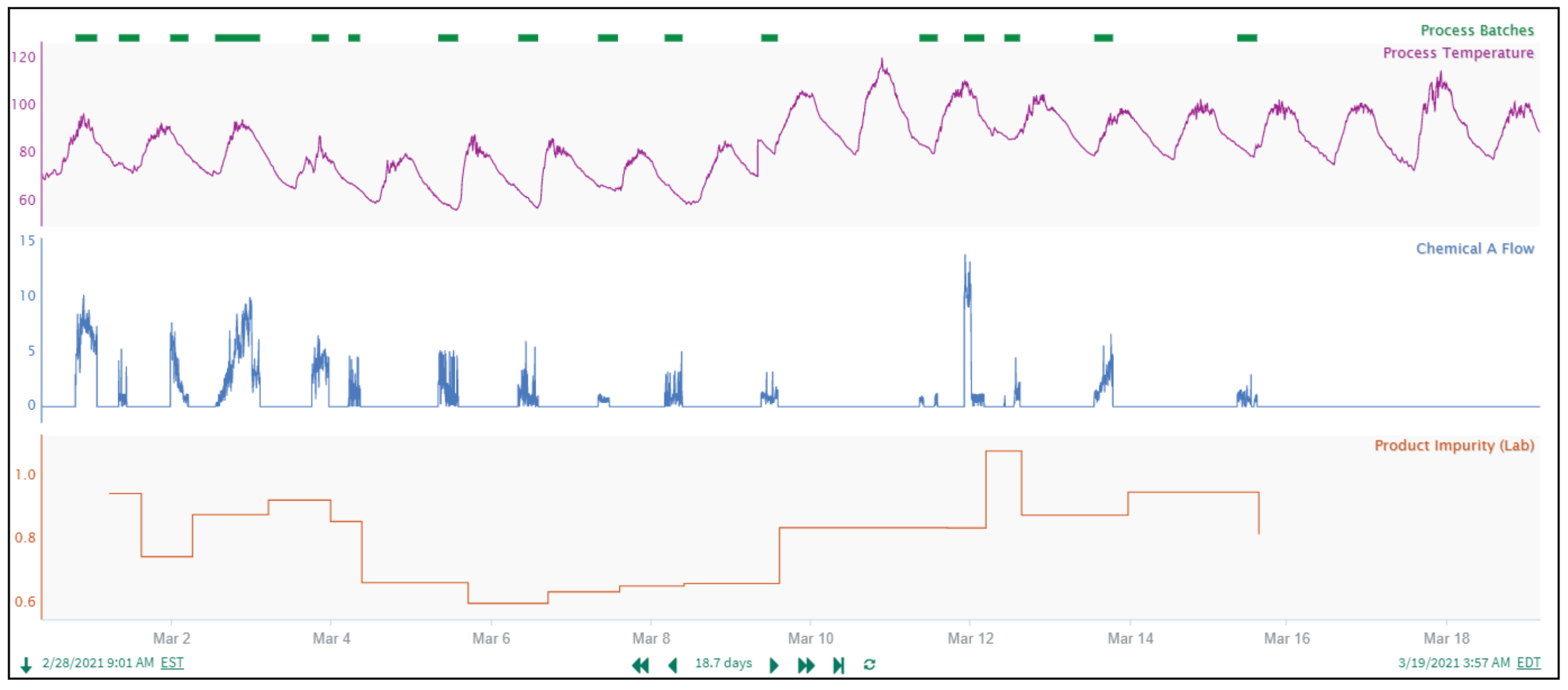
The “Product Impurity” lab results are always reported in the expected sequence after the Process Batches complete. This enables use of Seeq’s Composite Condition to create a common time basis for aligning process values with lab results. The Composite Condition tool joins the start of the “Process Batches” condition capsules (green capsules in Figure 7) to the end of the subsequent “Product Impurity Results Capsules” (brown capsules in Figure 7)), resulting in the “joined” yellow condition. With the Process Batches now linked to the correct Product Impurity Results, the Max Temperature per Batch, Total Chemical A per Batch, and Product Impurity are all aligned at the middle of each yellow capsule, and further calculations or prediction models can be confidently pursued.
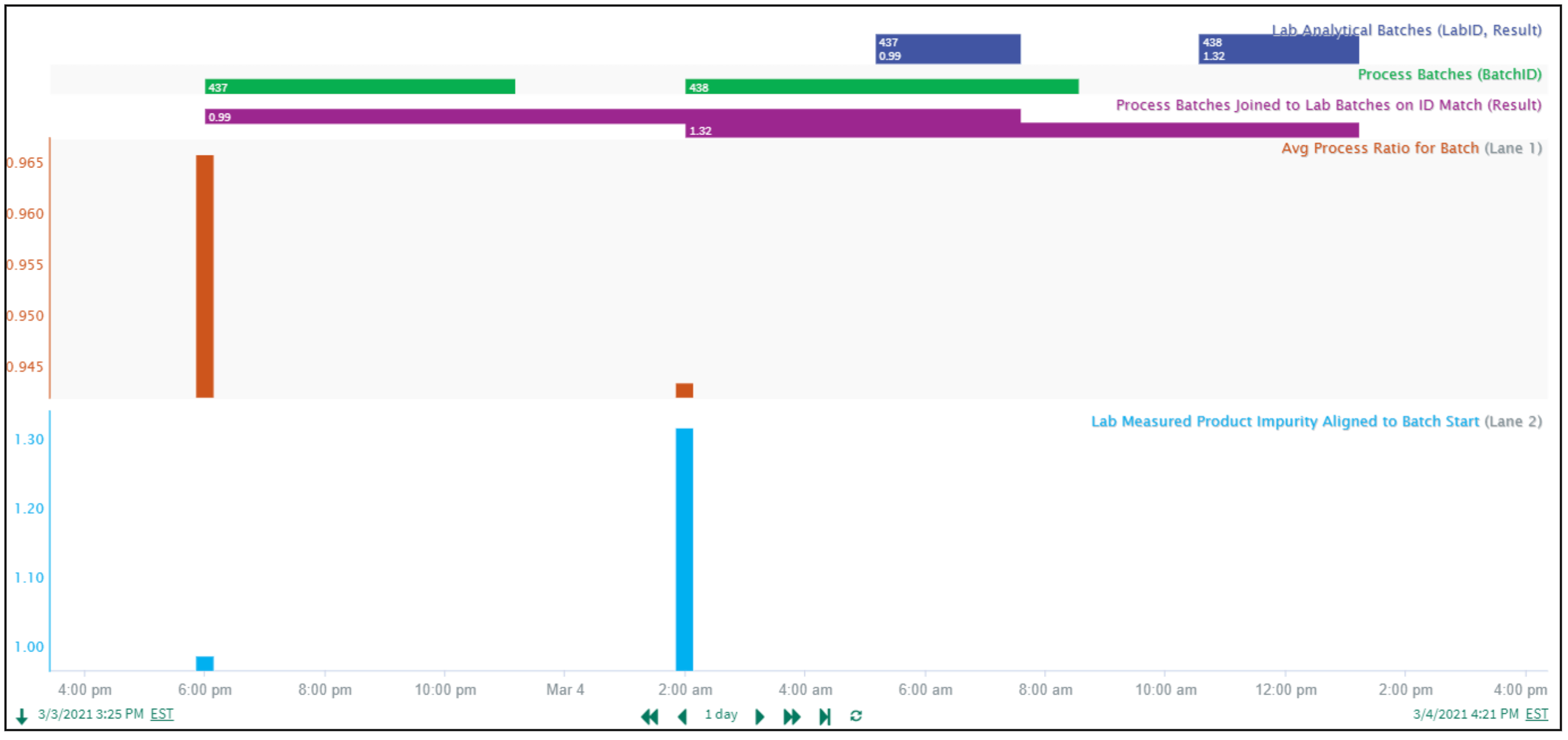
Let’s look at another common scenario in this use case category: when analytical results can be reported inconsistently or out of sequence with process batches, a more advanced condition join using Seeq Formula, based on a matching id value, may be needed. Here (Figure 8), the numeric batch id is a capsule property shared by the Process Batches and Lab Analytical Batches conditions. Using this batch id linkage, matching id capsules can be joined with a single line formula, and capsule properties from the separate process and lab conditions can be preserved, all courtesy of enhanced “capsule matching by property” functionality introduced in Seeq R56. As a result, we have now connected the lab measured product impurity result to each individual process batch, regardless of lab result timing, and with additional steps have aligned the Average Process Ratio and Lab Measured Product Impurity.
The ability to precisely align data values related to inherent time delays and process events saves significant time preparing data for calculations and modeling. It is essential to achieving accurate data driven process insights and, ultimately, leads to better process adjustments and more optimal process performance.
Do you have a use case like the ones described above? You can see all the details on the analytics tools and formulas needed for these use case categories described above in a post on seeq.org.
If you are ready to discuss how Seeq can improve your operations, please contact us to speak with one of our industry experts and schedule a demo today.