Our Investments, Your Outcomes: Shortening the Pharmaceutical Lifecycle to Provide Better, Faster Cures
3 Ways Seeq is Shortening the Pharmaceutical Lifecycle
The life sciences industry is booming, valued at 1.3 trillion dollars, with approximately 300 global companies delivering over 20,000 different lifesaving treatments, personalized medicines, gene therapies, and cures to patients annually. It may take just a moment for a patient to pop a pill or receive an injection, but these treatments typically require decades of research and billions of dollars to develop and bring to market. Drugs are often prepared for consumption by contract development manufacturing organizations (CDMOs) under stringent Food and Drug Administration (FDA) regulations.
While the drug development cycle is lengthy, the COVID-19 vaccine is a recent and notable exception. Requiring just over one year to develop and bring to market, its distribution was, and continues to be, a remarkable feat. At Seeq, we are striving to normalize this timeline and enable life sciences companies to shorten development and distribution cycles so patients around the world can access cures whenever there’s a need.
Using democratized and relevant data analytics, Seeq helps life sciences and pharmaceutical organizations accelerate time to market while ensuring product integrity. From targets and KPIs to materials and delivery, Seeq empowers its users with insights and knowledge-sharing capabilities throughout all stages of discovery and production.
Here are three areas where Seeq investments in the life sciences industry are improving outcomes and shortening development cycles.
1. Regulatory compliance in pharmaceutical industry
Process failure is the leading cause of drug shortages, but continued process verification (CPV) empowers teams to identify process losses early, reducing overall failure rates. Rather than relying on delayed lab results, teams can use online models to predict batch quality and yield based on variables like concentration, volume, and temperature. By identifying anomalies rapidly and early, and performing root cause analysis, they can adjust process parameters during batching, reducing energy usage and material waste, in addition to out-of-spec batches. This increased process integrity leads to faster time to market and less negative attention from the FDA.

Seeq is an effective CPV tool, and by connecting all necessary time-series data sources without duplicating or impacting data integrity, it provides users with access to a single source of truth among process historians, laboratory information management systems, manufacturing execution systems, enterprise resource planning systems, and others. Users can cleanse and contextualize data, adding batch, phase, and asset information to understand equipment performance for maintenance and calibration. Once that is set up, teams of engineers and data scientists can analyze the data using multiple methods including point-and-click statistical analysis tools, advanced AI- and ML-based models, and trend analysis tools. With real-time collaboration tools built into the software, it’s easy for teams to share insights, and proactively detect and interpret operational anomalies to avoid deviations, quality violations, and production downtime.
2. Agility in contract manufacturing
While many pharmaceutical companies conveniently outsource development and production to CDMOs, this outsourcing and capacity enhancing practice presents challenges when it comes to data sharing. CDMOs must provide raw manufacturing data to clients, and they often store this data in unique systems dedicated to each client to ensure only authorized access.
This data management process is typically executed manually, and it can take days or weeks to transfer all appropriate data between organizations. When a client pharma company receives the data, it’s often in the form of numerous spreadsheets, making it difficult to aggregate all data to visualize, perform analytics, and confirm overall results. With this model, client visibility is limited and delayed, restricting the ability to oversee supply chain, and to make process improvements between batches.
Seeq connects to disparate data sources, and it automatically aligns data in time for easy information comparison among various sources and profiles. From here, users can model and automate outputs to provide the desired data to the client, eliminating ongoing manual intervention, spreadsheets, and paper from the data transfer process. Because Seeq does not duplicate data, there is no need for users to revalidate its accuracy in a secondary database, empowering organizations to save time and preserve data integrity.
For example, a large biopharmaceutical company implemented Seeq to accelerate the lengthy and costly process of bringing a new medicine to market. By combining lab and pilot plant data to visualize trends and perform analytics, the company reduced its process development time to meet more clinical deadlines. They valued the timesavings and additional opportunities at a value over 1.5 million USD.
3. Knowledge management and quality by design
Every pharmaceutical manufacturing company has unique needs, each requiring different algorithms to address vertical and asset-specific issues across the value chain. Seeq’s “Build Your Own Algorithm” initiative enables users to create and use a portfolio of algorithms from multiple sources, rather than relying on a single vendor or platform. Pharma companies can develop algorithms with tools—such as Azure Machine Learning Studio, AWS SageMaker, Anaconda, and others—or deploy premade algorithms from third parties, including Amazon Lookout for Equipment and Azure AutoML.
Teams can also source new algorithms and Add-ons from Seeq and the broader community via our open-source gallery on GitHub. An Add-on is a tool used to operationalize an algorithm for additional team members, such as process engineers, so they can use the algorithm in their workflows without requiring support from a data scientist each time new data becomes available. Users can create custom Add-ons and integrate them into their Add-ons Directory in Workbench.
For example, a pharmaceutical company transformed its internally-developed and unsupervised learning algorithm for proactively detecting sensor drift into a Seeq Add-on. Previously, the workflow consisted of an on-site process engineering team manually uploading and cleansing data, exporting it to a data science team, processing it in a Python script, and then sending it back to the engineering team for analysis. Converting this process into a Seeq Add-on eliminated manual processing and back-and-forth, empowering the engineers and data scientists to spend more time collaborating and improving the model, rather than cleansing, preparing, and transferring data.
During the manufacturing process, pharmaceutical companies must maintain certain process parameters to ensure the batch meets stringent quality requirements. Critical parameters, such as temperature, must not deviate from a required range, and failure to comply results in a wasted batch, requiring rework and costing potentially millions of dollars in lost revenue.
To address this risk, a new approach called Quality by Design (QbD) enables organizations to quantitatively assess the process parameters that define batch quality, based on multiple variables and the ways they interact with each other. It utilizes experimental models to broaden the range of a single variable provided all other parameters are within range. QbD expands the process design space to reduce the number of quality deviations, saving organizations significant costs and lost production time.
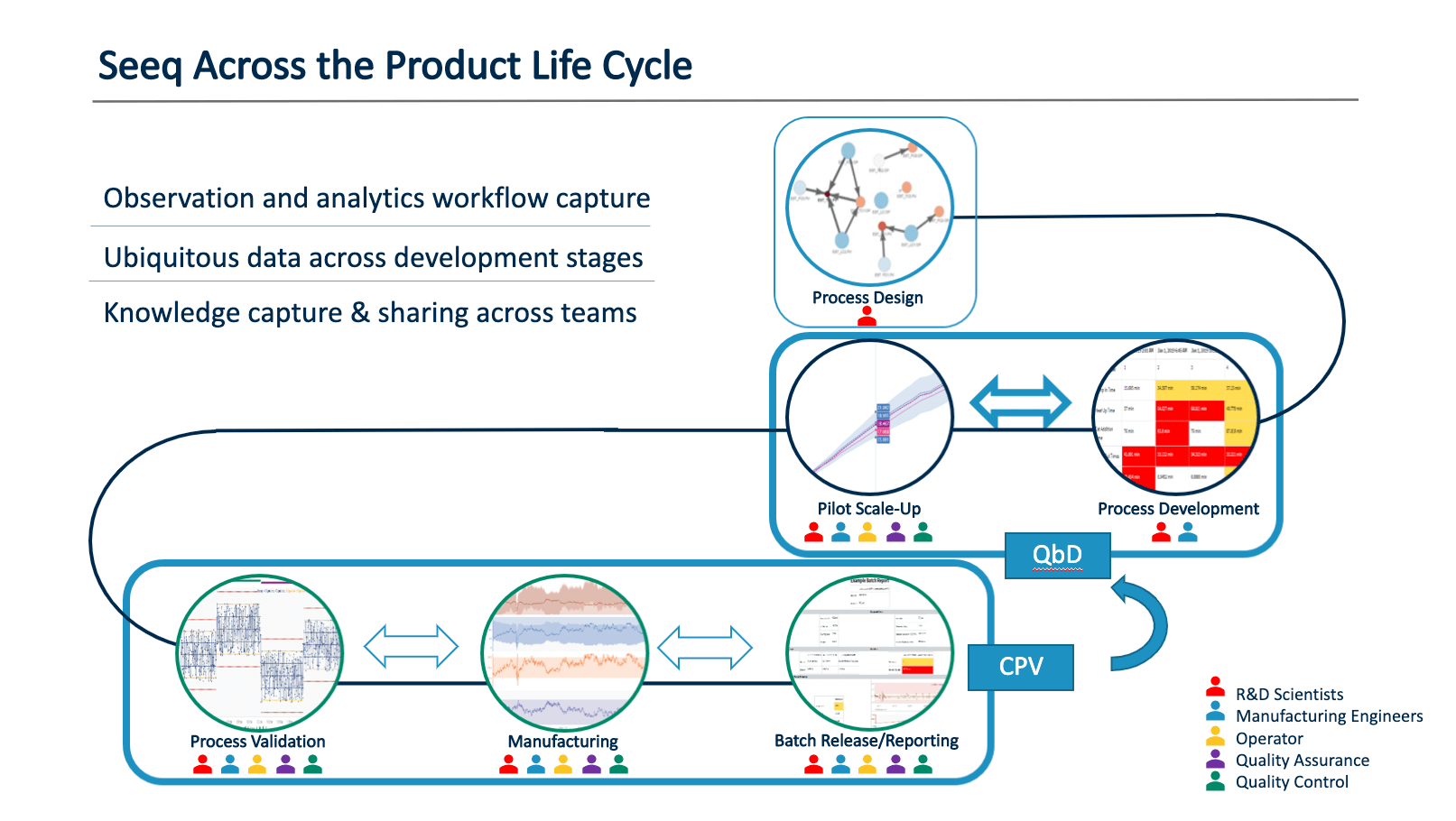
As a new drug is commercialized, its recipe and process parameters are transferred from research and development centers to commercial production plants. Oftentimes, important information can be lost in this transfer, but by using Seeq pharmaceutical organizations can confidently transfer this process design information in its entirety. This improves quality, and accelerates speed from development to commercial production, resulting in minimal quality deviations and increased cost savings.
At Seeq, we are committed to helping our pharmaceutical clients provide better, faster cures. To take a deeper dive into what we do for life sciences companies, explore our Pharmaceuticals & Life Sciences Analytics page, or the Bring Your Own Algorithm webinar.
New to Seeq? To see the technology in action, schedule a demo today.